HIV-1’s encounter with the host cell
Read full article on ScienceDirect.
Quantitative analyses reveal distinct sensitivities of the capture of HIV-1 primary viruses and pseudoviruses to broadly neutralizing antibodies
Text: Jiae Kim, Mangala Rao and Venigalla B. Rao
This work started by asking the question, what does the HIV-1 virus “see” on the host cell? What are the dynamics of communication between the host cell and HIV-1 at their first encounter? These interactions might be critical for subsequent virus entry and to design vaccines that can prevent HIV-1 transmission at the site of exposure.
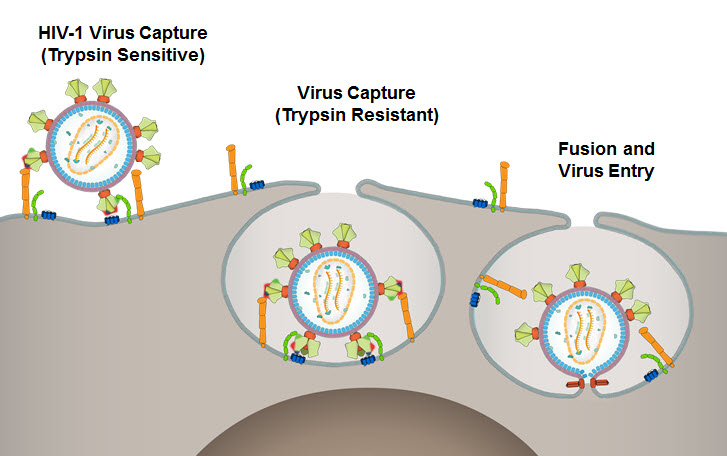
Our hypothesis was that the initial interactions would merely attach the HIV-1 virus to the host cell, allowing the virus to “seek” its primary and co-receptors, CD4 and CCR5/CXCR4. The probability of HIV-1 directly landing on a CD4 receptor or by a random search is rather low. The virus first would have to be captured by the cell through molecules that are easily accessible to the virus, which then facilitate the HIV-1-receptor/co-receptor interactions and entry. To study this and to resolve basic mechanistic questions on viral capture and entry, we embarked on designing a new virus capture assay.
This quantitative, high-throughput assay measures HIV-1 entry within minutes of exposure of the virus to host cells. To mimic natural transmission, we utilized primary viruses propagated in PBMCs to infect human T lymphoblastoid A3R5.7 cells. The number of copies of internalized viral RNA was quantified by qRT-PCR using primers for p24 or LTR genes. We found that the virus was rapidly converted to a trypsin-resistant form within 5 minutes of exposure. Surprisingly, this internalization was not inhibited by any of the broadly neutralizing antibodies (bNAbs). Primary viruses from different subtypes, an anti-CD4 antibody, soluble CD4, and even a cocktail of bNAbs, also gave similar results. The bNAbs and the anti-CD4 antibody strongly inhibited the production of progeny viruses at 48 hours, in agreement with the previous results, however the bNAbs had to be present before initial exposure. Another surprising finding was that in contrast to the primary viruses, capture of pseudoviruses and infectious molecular clones (IMCs) produced by transfection of HEK293 cells was inhibited by bNAbs.
As noted above, there were many surprises along the way with this study. We believe that we would not have observed these differences had we not used primary HIV-1. According to our model, the primary virus is captured by a cell-surface molecule(s) and internalized into a trypsin-resistant endocytic-type vesicle followed by interaction with CD4/CCR5 receptors and membrane fusion. Pseudoviruses and IMCs differ in this respect perhaps because the structure and composition of their envelopes are different.
These unexpected findings provide new insights into the mechanisms of HIV-1 transmission and strategies to prevent it. They imply that the vaccine-stimulated antibody responses should also block capture in order to effectively prevent HIV-1 transmission and establishment of early reservoirs. Our capture assay complements current neutralization approaches and could be exploited for better assessment of vaccine efficacy and for designing more effective prophylactic vaccines.